

Sterile Barrier System is a fundamental requirement for maintaining the safety and effectiveness of sterile medical devices. The innermost packaging material plays a crucial role as the cornerstone of this sterile barrier system. It provides physical protection, blocks icroorganisms and harmful substances to ensure the sterility and integrity of the packaged medical products during their shelf life,and the packaging material itself has biocompatibility. Therefore, medical packaging materials need to have excellent barrier and clean peel properties, high mechanical strength, and compatibility with most sterilization methods.
With a focus on patient safety, regulatory compliance, and product integrity, manufacturers are continually seeking materials that meet rigorous standards. Kingwills's Hypak™, has emerged as a preferred choice for sterile packaging in medical packaging industry. Its unique properties not only ensure optimal protection during transport and storage but also enhance the overall process of medical device production and use.
Flash spun Hypak™ is a high-density polyethylene fiber that is revered for its exceptional balance of strength, durability, and flexibility. This innovative material offers numerous advantages that make it a highly suitable option for sterile packaging:
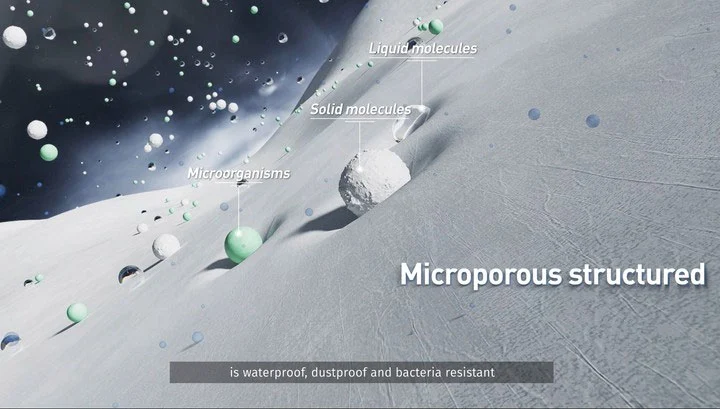
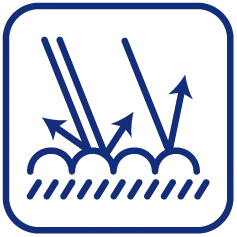
Breathability: The unique structureHypak™'s allows for micro-porosity, facilitating gas exchange while preventing the ingress of pathogens. This feature is particularly beneficial during sterilization processes, such as ethylene oxide (EtO) and steam sterilization, where the sterilant must penetrate the packaging before it is confined.
Barrier Properties: Thanks to the unique nano-sized fiber mesh structure constructed by flash-spinning technolo_x0002_gy, Hypak™effectively prevents bacteria, spores and other contaminating microorganisms from
penetrating into the medical device package, keeping it sterile until it is opened.
Durability: The strong filament fiber structure gives Hypak™ excellent mechanical properties, providing
reliable physical protec_x0002_tion for medical devices. Its excellent tear strength and puncture resistance minimize product recalls due to packaging breakage.
Compatibility with Sterilization Techniques: The excellent chemical stability of HDPE makes Hypak™ suitable for a wide range of conven_x0002_tional sterilization methods. The material can withstand sterilization
processes such as ethylene oxide (EtO), gamma radiation, electron beam, and steam (under controlled conditions). Hypak™ maintains stable microbi_x0002_al barrier and mechanical properties before and after sterilization .





 Kingwills International Limited
Kingwills International Limited